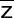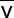Calculate too:
Interaction force of charged plates
| UniCalc |
|
RU | mobile version |
|
Find a formula by keywords |
Calculate own formula |
||||||||
|
Loading
|
|
|||||||||
Kinetic theory of gases |
Average number of molecule's encounters
|